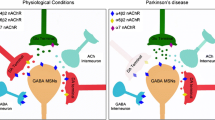
Abstract
Parkinson’s disease (PD), an age-related progressive neurodegenerative condition, is associated with loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), which results in motor deficits characterized by the following: akinesia, rigidity, resting tremor, and postural instability, as well as nonmotor symptoms such as emotional changes, particularly depression, cognitive impairment, gastrointestinal, and autonomic dysfunction. The most common treatment for PD is focused on dopamine (DA) replacement (e.g., levodopa = L-Dopa), which unfortunately losses its efficacy over months or years and can induce severe dyskinesia. Hence, more efficacious interventions without such adverse effects are urgently needed. In this review, following a general description of PD, potential novel therapeutic interventions for this devastating disease are examined. Specifically, the focus is on nicotine and nicotinic cholinergic system, as well as butyrate, a short chain fatty acid (SCFA), and fatty acid receptors.

Similar content being viewed by others
References
Almeida C, Oliveira R, Soares R, Barata P (2020) Influence of gut microbiota dysbiosis on brain function: a systematic review. Porto Biomed j 5(2):1–8
Alrafas HR, Busbee PB, Nagarkatti M (2019) Nagarkatti PS Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J Leukoc Biol 106(2):467–480
Anderson CC, Marentette JO, Rauniyar AK, Prutton K, Khatri M, Matheson C et al (2020) Maneb alters central carbon metabolism and thiol redox status in a toxicant model of Parkinson’s disease. Free Radic Biol Med S0891–5849(20):31641–31645
Andrade VM, Aschner M (2017) Marreilha Dos Santos AP. Neurotoxicity of Metal Mixtures Adv Neurobiol 18:227–265
Antkiewicz-Michaluk L (2002) Endogenous risk factors in Parkinson’s disease: dopamine and tetrahydroisoquinolines. Pol J Pharmcol 54:567–572
Aosaki T, Miura M, Suzuki T, Nishimura K, Masuda M (2010) Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr Gerontol Int 10(Suppl 1):S148–S157
Aradi SD, Hauser RA (2020) Medical management and prevention of motor complications in Parkinson’s disease. Neurotherapeutics 17(4):1339–1365
Assous M (2021) Striatal cholinergic transmission. Focus on nicotinic receptors' influence in striatal circuits. Eur J Neurosci. 53(8):2421–2442
Azimi M, Oemisch M, Womelsdorf T (2020) Dissociation of nicotinic α7 and α4/β2 sub-receptor agonists for enhancing learning and attentional filtering in nonhuman primates. Psychopharmacology 237(4):997–1010
Bagdas D, Gurun MS, Flood P, Papke RL, Damaj MI (2018) New insights on neuronal nicotinic acetylcholine receptors as targets for pain and inflammation: a focus on α7 nAChRs. Curr Neuropharmacol 16:415–425
Baizabal-Carvallo JF, Alonso-Juarez M (2020) The link between gut dysbiosis and neuroinflammation in Parkinson’s disease. Neuroscience 432:160–173
Barreto GE, Iarkov A, Moran VE (2015) Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson’s disease. Front Aging Neurosci 6:340
Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM (2019) Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio. 10(1):e02566–18
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3(12):1301–1306
Bjørklund G, Dadar M, Anderson G, Chirumbolo S, Maes M (2020) Preventive treatments to slow substantia nigra damage and parkinson’s disease progression: a critical perspective. Review Pharmacol Res 161:105065
Blesa J, Przedborski S (2014) Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front Neuroanat 8:155
Bolognini D, Tobin AB, Milligan G, Moss CE (2016) The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol 89(3):388–398
Bordia T, McGregor M, Papke RL, Decker MW, McIntosh JM, Quik M (2015) The α7 nicotinic receptor agonist ABT-107 protects against nigrostriatal damage in rats with unilateral 6-hydroxydopamine lesions. Exp Neurol 263:277–284
Bordia T, Perez XA (2019) Cholinergic control of striatal neurons to modulate L-dopa-induced dyskinesias. Eur J Neurosci 49(6):859–868
Cantu-Jungles TM, Rasmussen HE, Hamaker BR (2019) Potential of prebiotic butyrogenic fibers in Parkinson’s disease. Front Neurol 10:663
Caspani G, Kennedy S, Foster JA, Swann J (2019) Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microb Cell 6(10):454–481
Changeux JP (2018) The nicotinic acetylcholine receptor: a typical “allosteric machine.” Philos Trans R Soc Lond B Biol Sci 373(1749):20170174
Chi L, Mahbub R, Gao B, Bian X, Tu P, Ru H et al (2017) Nicotine alters the gut microbiome and metabolites of gut-brain interactions in a sex-specific manner. Chem Res Toxicol 30(12):2110–2119
Cirstea MS, Yu AC, Golz E, Sundvick K, Kliger D, Radisavljevic N et al (2020) Microbiota composition and metabolism are associated with gut function in Parkinson’s disease. Mov Disord 35(7):1208–1217
Clarke PB, Pert A (1985) Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res 348(2):355–358
Conti AA, Tolomeo S, Steele JD, Baldacchino AM (2020) Severity of negative mood and anxiety symptoms occurring during acute abstinence from tobacco: a systematic review and meta-analysis. Neurosci Biobehav Rev 115:48–63
Copeland RL Jr, Das JR, Kanaan YM, Taylor RE, Tizabi Y (2007) Antiapoptotic effects of nicotine in its protection against salsolinol-induced cytotoxicity. Neurotox Res 12(1):61–69
Cruz-Pereira JS, Cryan JF (2020) In need of a quorum: from microbes to mood via the immune system. Am J Psychiatry 177(10):895–897. https://doi.org/10.1176/appi.ajp.2020.20081182
Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG (2020) The gut microbiome in neurological disorders. Lancet Neurol 19(2):179–194
Dani JA (2015) Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int Rev Neurobiol 124:3–19
Dinan TG, Cryan JF (2020) Gut microbiota: a missing link in psychiatry. World Psychiatry 19(1):111–112
Dinter E, Saridaki T, Diederichs L, Reichmann H, Falkenburger BH (2020) Parkinson’s disease and translational research. Transl Neurodegener 9(1):43
Dong Y, Bi W, Zheng K, Zhu E, Wang S, Xiong Y et al (2020) Nicotine prevents oxidative stress-induced hippocampal neuronal injury through α7-nAChR/Erk1/2 signaling pathway. Front Mol Neurosci 13:557647
Dorszewska J, Kowalska M, Prendecki M, Piekut T, Kozlowska J, Kozubski W (2021) Oxidative stress factors in Parkinson’s disease. Neural Regen Res 16(7):1383–1391
Featherstone RE, Siegel SJ (2015) The Role of Nicotine in Schizophrenia. Int Rev Neurobiol 124:23–78
Fujita A, Fujita Y, Pu Y, Chang L, Hashimoto K (2020) MPTP-induced dopaminergic neurotoxicity in mouse brain is attenuated after subsequent intranasal administration of (R)-ketamine: a role of TrkB signaling. Psychopharmacology 237(1):83–92
Funakohi-Tago M, Sakata T, Fujiwara S, Sakakura A, Sugai T, Tago K et al (2018) Hydroxytyrosol butyrate inhibits 6-OHDA-induced apoptosis through activation of the Nrf2/HO-1 axis in SH-SY5Y cells. Eur J Pharmacol 834:246–256
Gandelman JA, Newhouse P, Taylor WD (2018) Nicotine and networks: potential for enhancement of mood and cognition in late-life depression. Neurosci Biobehav Rev 84:289–298
Ganz AB, Beker N, Hulsman M, Sikkes S, Netherlands Brain Bank Scheltens P, Smit AB et al (2018) Neuropathology and cognitive performance in self-reported cognitively healthy centenarians. Acta Neuropathol Commun 6(1):64
Genovese I, Giamogante F, Barazzuol L, Battista T, Fiorillo A, Vicario M et al (2020) Sorcin is an early marker of neurodegeneration, Ca2+dysregulation and endoplasmic reticulum stress associated to neurodegenerative diseases. Cell Death Dis 11(10):861
Getachew B, Csoka AB, Aschner M, Tizabi Y (2019a) Nicotine protects against manganese and iron-induced toxicity in SH-SY5Y cells: implication for Parkinson’s disease. Neurochem Int 124:19–24
Getachew B, Reyes RE, Davies DL, Tizabi Y (2019b) Moxidectin effects on gut microbiota of Wistar-Kyoto rats: relevance to depressive-like behavior. Clin Pharmacol Transl Med 3(1):134–142
Getachew B, Tizabi Y (2019) Antidepressant effects of moxidectin, an antiparasitic drug, in a rat model of depression. Behav Brain Res 376:112220
Getachew B, Csoka AB, Bhatti A, Copeland RL, Tizabi Y (2020) Butyrate protects against salsolinol-induced toxicity in SH-SY5Y cells: implication for Parkinson’s disease. Neurotox Res 38(3):596–602
Ghosh SK, Perrine SP, Williams RM, Faller DV (2012) Histone deacetylase inhibitors are potent inducers of gene expression in latent EBV and sensitize lymphoma cells to nucleoside antiviral agents. Blood 119(4):1008–1017
Giorgi C, Bouhamida E, Danese A, Previati M, Pinton P, Patergnani S (2021) relevance of autophagy and mitophagy dynamics and markers in neurodegenerative diseases. Biomedicines 9(2):149
Grün D, Zimmer VC, Kauffmann J, Spiegel J, Dillmann U, Schwiertz A et al (2020) Impact of oral COMT-inhibitors on gut microbiota and short chain fatty acids in Parkinson’s disease. Parkinsonism Relat Disord 70:20–22
Gundersen BB, Blendy JA (2009) Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology 57(1):67–74
Hahn B, Harvey AN, Concheiro-Guisan M, Huestis MA, Ross TJ, Stein EA (2020) Nicotinic receptor modulation of the default mode network. Psychopharmacology 238(2):589–597
Han C, Lu Y, Cheng H, Wang C, Chan P (2020a) The impact of long-term exposure to ambient air pollution and second-hand smoke on the onset of Parkinson disease: a review and meta-analysis. Public Health 179:100–110
Han T, Wang Q, Lai R, Zhang D, Diao Y, Yin Y (2020b) Nicotine induced neurocognitive protection and anti-inflammation effect by activating α 4β 2 nicotinic acetylcholine receptors in ischemic rats. Nicotine Tob Res 22(6):919–924
Harms AS, Ferreira SA, Romero-Ramos M (2021) Periphery and brain, innate and adaptive immunity in Parkinson's disease. Acta Neuropathol. Epub ahead of print
Hernán MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ (2002) A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol 52(3):276–284
Hou Y, Li X, Liu C, Zhang M, Zhang X, Ge S, Zhao L (2021) Neuroprotective effects of short-chain fatty acids in MPTP induced mice model of Parkinson's disease. Exp Gerontol 111376
Huang C, Ma W, Luo Q, Shi L, Xia Y, Lao C et al (2019) Iron overload resulting from the chronic oral administration of ferric citrate induces parkinsonism phenotypes in middle-aged mice. Aging (albany NY) 11(21):9846–9861
Hudson BD, Christiansen E, Murdoch H, Jenkins L, Hansen AH, Madsen O et al (2014) Complex pharmacology of novel allosteric free fatty acid 3 receptor ligands. Mol Pharmacol 86(2):200–210
Hurley LL, Tizabi Y (2013) Neuroinflammation, neurodegeneration, and depression. Neurotox Res 23(2):131–144
Hustad E, Aasly JO (2020) Clinical and imaging markers of prodromal Parkinson’s disease. Front Neurol 11:395. https://doi.org/10.3389/fneur.2020.00395
Indrieri A, Pizzarelli R, Franco B, De Leonibus E (2020) Dopamine, alpha-synuclein, and mitochondrial dysfunctions in parkinsonian eyes. Front Neurosci 14:567129
Ivleva I, Pestereva N, Zubov A, Karpenko M (2020) Intranasal exposure of manganese induces neuroinflammation and disrupts dopamine metabolism in the striatum and hippocampus. Neurosci Lett 738:135344
Kaji I, Akiba Y, Furuyama T, Adelson DW, Iwamoto K et al (2018) Free fatty acid receptor 3 activation suppresses neurogenic motility in rat proximal colon. Neurogastroenterol Motil 30(1):10
Kalamida D, Poulas K, Avramopoulou V, Fostieri E, Lagoumintzis G, Lazaridis K et al (2007) Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity. FEBS J 274(15):3799–3845
Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG (2020) The role of the gut microbiome in the development of schizophrenia. Schizophr Res S0920–9964(20):30086–30094
Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB et al (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30:1351–1360
Klein C, Westenberger A (2012) Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med 2(1):a008888
Koukouli F, Changeux JP (2020) Do nicotinic receptors modulate high-order cognitive processing? Trends Neurosci S0166–2236(20):30125–30129
Kouli A, Torsney K M, Kuan WL (2018) Parkinson’s disease: etiology, neuropathology, and pathogenesis. In: Stoker TB, Greenland JC, editors. Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]. Brisbane (AU): Codon Publications, Chapter 1
Koutzoumis DN, Vergara M, Pino J, Buddendorff J, Khoshbouei H, Mandel RJ et al (2020) Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson’s disease. Exp Neurol 325:113159
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219(4587):979–980
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373(9680):2055–2066
Lemay S, Chouinard S, Blanchet P, Masson H, Soland V, Beuter A et al (2004) Lack of efficacy of a nicotine transdermal treatment on motor and cognitive deficits in Parkinson’s disease. Prog Neuropsychopharm Biol Psychiatry 28:31–39
Li X, Shen L, Hua T, Liu ZJ (2020a) Structural and functional insights into cannabinoid receptors. Trends Pharmacol Sci 41(9):665–677
Li D, Croft DP, Ossip DJ, Xie Z (2020b) The association between statewide vaping prevalence and COVID-19. Prev Med Rep 20:101254
Li X, Li W, Liu G, Shen X, Tang Y (2015) Association between cigarette smoking and Parkinson’s disease: a meta-analysis. Arch Gerontol Geriatr 61(3):510–516
Lieberman A, Deep A, Olson MC, Smith Hussain V, Frames CW, McCauley M et al (2019) Falls when standing, falls when walking: different mechanisms, different outcomes in Parkinson disease. Cureus 11(8):e5329
Liu C (2020) Targeting the cholinergic system in Parkinson’s disease. Acta Pharmacol Sin 41(4):453–463
Liu J, Wang F, Liu S, Du J, Hu X, Xiong J et al (2017a) Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci 381:176–181
Liu Z, Roosaar A, Axéll T, Ye W (2017b) Tobacco use, oral health, and risk of Parkinson’s disease. Am J Epidemiol 185(7):538–545
Liu W, Wang B, Xiao Y, Wang D, Chen W (2020) Secondhand smoking and neurological disease: a meta-analysis of cohort studies. Rev Environ Health. Epub ahead of print
Lungba RM, Khan SZA, Ajibawo-Aganbi U, Perez Bastidas MV, Veliginti S, Saleem S et al (2020) The role of the gut microbiota and the immune system in the development of autism. Cureus 12(10):e11226
Ma C, Molsberry S, Li Y, Schwarzschild M, Ascherio A, Gao X (2020) Dietary nicotine intake and risk of Parkinson disease: a prospective study. Am J Clin Nutr 112(4):1080–1087
Maiti P, Manna J, Dunbar GL (2017) Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl Neurodegener 6:28
Maruyama W, Yi H, Takahashi T, Shimazu S, Ohde H, Yoneda F et al (2004) Neuroprotective function of R-(−)-1-(benzofuran-2-yl)-2-propylaminopentane, [R-(−)-BPAP], against apoptosis induced by N-methyl(R)salsolinol, an endogenous dopaminergic neurotoxin, in human dopaminergic neuroblastoma SH-SY5Y cells. Life Sci 75:107–117
McKnight S, Hack N (2020) Toxin-induced parkinsonism. Neurol Clin 38(4):853–865
Meng L, YuanX, Xuebing Cao X, Zhang Z (2019) The gut-brain axis in the pathogenesis of Parkinson’s disease. Brain Sci Adv 5(2):73–81
Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL et al (2019) Gait impairments in Parkinson’s disease. Lancet Neurol 18(7):697–708
Mitra S, Khatri SN, Maulik M, Bult-Ito A, Schulte M (2020) Allosterism of nicotinic acetylcholine receptors: therapeutic potential for neuroinflammation underlying brain trauma and degenerative disorders. Int J Mol Sci 21(14):4918
Mravec B (2006) Salsolinol, a derivate of dopamine, is a possible modulator of catecholaminergic transmission: a review of recent developments. Physiological Research / Academia Scientiarum Bohemoslovaca 55:353–364
Naoi M, Maruyama W, Nagy GM (2004) Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: occurrence, metabolism and function in human brains. Neurotoxicology 25:193–204
Ndayisaba A, Kaindlstorfer C, Wenning GK (2019) Iron in neurodegeneration - cause or consequence? Front Neurosci 13:180. https://doi.org/10.3389/fnins.2019.00180
Nuzum ND, Loughman A, Szymlek-Gay EA, Hendy A, Teo WP, Macpherson H (2020) Gut microbiota differences between healthy older adults and individuals with Parkinson’s disease: a systematic review. Neurosci Biobehav Rev 112:227–241
O’Connor R, van De Wouw M, Moloney GM, Ventura-Silva AP, O’Riordan K, Golubeva AV et al (2021) Strain differences in behaviour and immunity in aged mice: relevance to autism. Behav Brain Res 399:113020
O’Reilly EJ, McCullough ML, Chao A, Henley SJ, Calle EE, Thun MJ et al (2005) Smokeless tobacco use and the risk of Parkinson’s disease mortality. Mov Disord 20(10):1383–1384
Osborn TM, Hallett PJ, Schumacher JM, Isacson O (2020) Advantages and recent developments of autologous cell therapy for Parkinson’s disease patients. Front Cell Neurosci 14:58
Paiva I, Pinho R, Pavlou MA, Hennion M, Wales P, Schütz AL et al (2017) Sodium butyrate rescues dopaminergic cells from alpha-synuclein-induced transcriptional deregulation and DNA damage. Hum Mol Genet 26(12):2231–2246
Papke RL, Lindstrom JM (2020) Nicotinic acetylcholine receptors: conventional and unconventional ligands and signaling. Neuropharmacology 168:108021
Parra I, Martínez I, Ramírez-García G, Tizabi Y, Mendieta L (2020) Differential Effects of LPS and 6-OHDA on microglia’s morphology in rats: implications for inflammatory model of Parkinson’s disease. Neurotox Res 37(1):1–11
Pavia CS, Plummer MM (2020) Clinical implications of nicotine as an antimicrobial agent and immune modulator. Biomed Pharmacother 129:110404
Peres TV, Schettinger MR, Chen P, Carvalho F, Avila DS, Bowman AB et al (2016) Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol 17(1):57
Perez XA (2015) Preclinical evidence for a role of the nicotinic cholinergic system in Parkinson’s disease. Neuropsychol Rev 25(4):371–383
Prenger MTM, Madray R, Van Hedger K, Anello M, MacDonald PA (2020) Social symptoms of Parkinson’s disease. Parkinsons Dis 8846544
Qiao CM, Sun MF, Jia XB, Li Y, Zhang BP, Zhao LP et al (2020a) Sodium butyrate exacerbates Parkinson’s disease by aggravating neuroinflammation and colonic inflammation in MPTP-induced mice model. Neurochem Res 45(9):2128–2142
Qiao CM, Sun MF, Jia XB, Shi Y, Zhang BP, Zhou ZL et al (2020b) Sodium butyrate causes α-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp Cell Res 387(1):111772
Qiu J, Liu R, Ma Y, Li Y, Chen Z, He H et al (2020) Lipopolysaccharide-induced depression-like behaviors is ameliorated by sodium butyrate via inhibiting neuroinflammation and oxido-nitrosative stress. Pharmacology 105(9–10):550–560
Qualls Z, Brown D, Ramlochansingh C, Hurley LL, Tizabi Y (2014) Protective effects of curcumin against rotenone and salsolinol induced toxicity: implications for Parkinson’s disease. Neurotox Res 25(1):81–89
Quik M, Bordia T, Zhang D, Perez XA (2015) Nicotine and nicotinic receptor drugs: potential for Parkinson’s disease and drug-induced movement disorders. Int Rev Neurobiol 124:247–271
Quik M, Boyd JT, Bordia T, Perez X (2019) Potential therapeutic application for nicotinic receptor drugs in movement disorders. Nicotine Tob Res 21(3):357–369
Rani L, Mondal AC (2021) Unravelling the role of gut microbiota in Parkinson’s disease progression: Pathogenic and therapeutic implications. Neurosci Res S0168–0102(21):00004–3. Epub ahead of print
Reglodi D, Renaud J, Tamas A, Tizabi Y, Socías SB, Del-Bel E et al (2017) Novel tactics for neuroprotection in Parkinson’s disease: role of antibiotics, polyphenols and neuropeptides. Prog Neurobiol 155:120–148
Ritz B, Ascherio A, Checkoway H, Marder KS, Nelson LM, Rocca WA et al (2007) Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol 64(7):990–997
Russo R, Cristiano C, Avagliano C, De Caro C, La Rana G, Raso GM et al (2018) Gut-brain axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr Med Chem 25(32):3930–3952
Ruszkiewicz JA, Zhang Z, Gonçalves FM, Tizabi Y, Zelikoff JT, Aschner M (2020) Neurotoxicity of e-cigarettes. Food Chem Toxicol 38:111245
Ryan RE, Ross SA, Drago J, Loiacono RE (2001) Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br J Pharmacol 132(8):1650–1656
Said H, Akiba Y, Narimatsu K, Maruta K, Kuri A, Iwamoto KI et al (2017) FFA3 activation stimulates duodenal bicarbonate secretion and prevents NSAID-induced enteropathy via the GLP-2 pathway in rats. Dig Dis Sci 62(8):1944–1952
Sampathkumar SG, Jones MB, Meledeo MA, Campbell CT, Choi SS, Hida K et al (2006) Targeting glycosylation pathways and the cell cycle: sugar-dependent activity of butyrate-carbohydrate cancer prodrugs. Chem Biol 13(12):1265–1275
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167(1469–80):e12
Scarduzio M, Zimmerman CN, Jaunarajs KL, Wang Q, Standaert DG, McMahon LL (2017) Strength of cholinergic tone dictates the polarity of dopamine D2 receptor modulation of striatal cholinergic interneuron excitability in DYT1 dystonia. Exp Neurol 295:162–175
Schneider JS, Marshall CA, Keibel L, Snyder NW, Hill MP, Brotchie JM et al (2021) A novel dopamine D3R agonist SK609 with norepinephrine transporter inhibition promotes improvement in cognitive task performance in rodent and non-human primate models of Parkinson’s disease. Exp Neurol 335:113514
Scholz J, Klingemann I, Moser A (2004) Increased systemic levels of norsalsolinol derivatives are induced by levodopa treatment and do not represent biological markers of Parkinson’s disease. J Neurol Neurosurg Psychiatry 75(4):634–636
Searles Nielsen S, Gallagher LG, Lundin JI, Longstreth WT Jr, Smith-Weller T, Franklin GM et al (2012) Environmental tobacco smoke and Parkinson’s disease. Mov Disord 27(2):293–296
Seoane-Collazo P, Diéguez C, Nogueiras R, Rahmouni K, Fernández-Real JM, López M (2021) Nicotine’s actions on energy balance: Friend or foe? Pharmacol Ther 219:107693
Sharma S, Moon CS, Khogali A, Haidous A, Chabenne A, Ojo C et al (2013) Biomarkers in Parkinson’s disease (recent update). Neurochem Int 63(3):201–229
Shi L, Huang C, Luo Q, Xia Y, Liu W, Zeng W et al (2020) Clioquinol improves motor and non-motor deficits in MPTP-induced monkey model of Parkinson’s disease through AKT/mTOR pathway. Aging (albany NY) 12(10):9515–9533
Shi L, Huang C, Luo Q, Rogers E, Xia Y, Liu W et al (2019) The association of iron and the pathologies of Parkinson’s diseases in MPTP/MPP+-induced neuronal degeneration in non-human primates and in cell culture. Front Aging Neurosci 11:215
Shimohama S, Kawamata J (2018) Roles of nicotinic acetylcholine receptors in the pathology and treatment of Alzheimer’s and Parkinson’s diseases. 2018 Apr 4 A Akaike S Shimohama Y Misu Eds Nicotinic Acetylcholine Receptor Signaling in Neuroprotection [Internet] Springer Singapore
Shin C, Lim Y, Lim H, Ahn TB (2020) Plasma short-chain fatty acids in patients with Parkinson’s disease. Mov Disord 35(6):1021–1027
Simon DK, Tanner CM, Brundin P (2020) Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med 36(1):1–12
Song Y, Wang ZY, Jin YY, Guo J (2019) Association between dopamine receptor D2 TaqIA polymorphism and Parkinson disease risk: a meta-analysis. Int J Clin Exp Pathol 12(9):3165–3170
Srivastav S, Neupane S, Bhurtel S, Katila N, Maharjan S, Choi H et al (2019) Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J Nutr Biochem 69:73–86
St Laurent R, O'Brien LM, Ahmad ST (2013) Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson's disease. Neuroscience 246:382-90 Stoker TB, Barker RA (2020) Recent developments in the treatment of Parkinson’s disease. F1000Research 9:862
Stoker TB, Torsney KM, Barker RA (2018) Emerging treatment approaches for Parkinson’s disease. Front Neurosci 12:693
Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology (Berl). 117(1):2–10; discussion 14–20
Storch A, Ott S, Hwang YI, Ortmann R, Hein A, Frenzel S et al (2002) Selective dopaminergic neurotoxicity of isoquinoline derivatives related to Parkinson’s disease: studies using heterologous expression systems of the dopamine transporter. Biochem Pharmacol 63:909–920
Sui Y, Tian Y, Ko WKD, Wang Z, Jia F, Horn A et al (2021) Deep brain stimulation initiative: toward innovative technology, new disease indications, and approaches to current and future clinical challenges in neuromodulation therapy. Front Neurol 11:597451
Sy MAC, Fernandez HH (2020) Pharmacological treatment of early motor manifestations of Parkinson disease (PD). Neurotherapeutics 17(4):1331–1338
Szentirmai É, Millican NS, Massie AR, Kapás L (2019) Butyrate, a metabolite of intestinal bacteria, enhances sleep. Sci Rep 9:7035
Tao Y, Vermilyea SC, Zammit M, Lu J, Olsen M, Metzger JM et al (2021) Autologous transplant therapy alleviates motor and depressive behaviors in parkinsonian monkeys. Nat Med 27(4):632–639
Tikhonova IG (2017) Application of GPCR structures for modelling of free fatty acid receptors. Handb Exp Pharmacol 236:57–77
Terry AV Jr, Callahan PM (2020) α7 nicotinic acetylcholine receptors as therapeutic targets in schizophrenia: Update on animal and clinical studies and strategies for the future. Neuropharmacology 170:108053
Tizabi Y (2016) Duality of antidepressants and neuroprotectants. Neurotox Res 30(1):1–13
Tizabi Y, Getachew B (2017) Nicotinic receptor intervention in Parkinson’s disease: future directions. Cin Pharm Transl Med 1:1–7
Tizabi Y, Getachew B, Csoka AB, Manaye KF, Copeland RL (2019) Novel targets for parkinsonism-depression comorbidity. Prog Mol Biol Transl Sci 167:1–24
Tizabi Y, Getachew B, Copeland RL, Aschner M (2020) Nicotine and the nicotinic cholinergic system in COVID-19. FEBS j 287(17):3656–3663
Tran J, Anastacio H, Bardy C (2020) Genetic predispositions of Parkinson’s disease revealed in patient-derived brain cells. npj Parkinsons Dis. 6:8
Unger MM, Spiegel J, Unger MM, Spiegel J, Dillmann KU, Grundmann D et al (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72
Valentine G, Sofuoglu M (2018) Cognitive effects of nicotine: recent progress. Curr Neuropharmacol 16(4):403–414
Valvassori SS, Varela RB, Arent CO, Dal-Pont GC, Bobsin TS, Budni J et al (2014) Sodium butyrate functions as an antidepressant and improves cognition with enhanced neurotrophic expression in models of maternal deprivation and chronic mild stress. Curr Neurovasc Res 11(4):359–366
Vega JN, Albert KM, Mayer IA, Taylor WD, Newhouse PA (2019) Nicotinic treatment of post-chemotherapy subjective cognitive impairment: a pilot study. J Cancer Surviv 13(5):673–686
Vetel S, Foucault-Fruchard L, Tronel C, Buron F, Vergote J, Bodard S et al (2021) Neuroprotective and anti-inflammatory effects of a therapy combining agonists of nicotinic α7 and σ1 receptors in a rat model of Parkinson’s disease. Neural Regen Res 16:1099–1104
Vieregge A, Sieberer M, Jacobs H, Hagenah JM, Vieregge P (2001) Transdermal nicotine in PD: a randomized, double-blind, placebo-controlled study. Neurol 57:1032–1035
Villageliú DN, Borts DJ, Lyte M (2018) Production of the neurotoxin salsolinol by a gut-associated bacterium and its modulation by alcohol. Front Microbiol 9:3092
Voon SM, Ng KY, Chye SM, Ling APK, Voon KGL, Yap YJ et al (2020) The mechanism of action of salsolinol in brain: implications in Parkinson’s disease. CNS Neurol Disord Drug Targets 19(10):725–740
Wills L, Kenny PJ (2021) Addiction-related neuroadaptations following chronic nicotine exposure. J Neurochem. https://doi.org/10.1111/jnc.15356. Epub ahead of print. PMID: 33742685
Witt O, Monkemeyer S, Rönndahl G, Erdlenbruch B, Reinhardt D, Kanbach K et al (2003) Induction of fetal hemoglobin expression by the histone deacetylase inhibitor apicidin. Blood 101(5):2001–2007
Wittenberg RE, Wolfman SL, De Biasi M, Dani JA (2020) Nicotinic acetylcholine receptors and nicotine addiction: a brief introduction. Neuropharmacology 177:108256
Xicoy H, Wieringa B, Martens GJ (2017) The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol Neurodegener 12(1):10
Yang F, Pedersen NL, Ye W, Liu Z, Norberg M, Forsgren L et al (2017) Moist smokeless tobacco (Snus) use and risk of Parkinson’s disease. Int J Epidemiol 46:872–880
Yoo CB, Jones PA (2006) Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov 5(1):37–50
Zeng XS, Geng WS, Jia JJ, Chen L, Zhang PP (2018) Cellular and molecular basis of neurodegeneration in parkinson disease. Front Aging Neurosci 10:109
Zheng X, Chen X, Guo M, Ali S, Huang Y, Sun F et al (2018) Changes in salsolinol production and salsolinol synthase activity in Parkinson’s disease model. Neurosci Lett 673:39–43
Zheng M, Chen M, Liu C, Fan Y, Shi D (2021) Alkaloids extracted from Uncaria rhynchophylla demonstrate neuroprotective effects in MPTP-induced experimental parkinsonism by regulating the PI3K/Akt/mTOR signaling pathway. J Ethnopharmacol 266:113451
Funding
Supported by NIH/NIAAA R03AA022479 and Howard University College of Medicine Bridge Funds and Pilot Study Awards Program (BFPSAP) 2020–2021 (YT) and NIH/NIEHS R01ES10563 and R01ES07331 (MA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tizabi, Y., Getachew, B. & Aschner, M. Novel Pharmacotherapies in Parkinson’s Disease. Neurotox Res 39, 1381–1390 (2021). https://doi.org/10.1007/s12640-021-00375-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00375-5