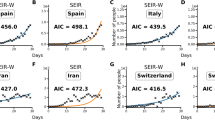
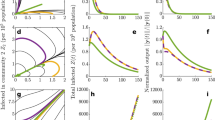
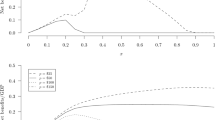
Abstract
Population-level analyses often use average quantities to describe heterogeneous systems, particularly when variation does not arise from identifiable groups1,2. A prominent example, central to our current understanding of epidemic spread, is the basic reproductive number, R0, which is defined as the mean number of infections caused by an infected individual in a susceptible population3,4. Population estimates of R0 can obscure considerable individual variation in infectiousness, as highlighted during the global emergence of severe acute respiratory syndrome (SARS) by numerous ‘superspreading events’ in which certain individuals infected unusually large numbers of secondary cases5,6,7,8,9,10. For diseases transmitted by non-sexual direct contacts, such as SARS or smallpox, individual variation is difficult to measure empirically, and thus its importance for outbreak dynamics has been unclear2,10,11. Here we present an integrated theoretical and statistical analysis of the influence of individual variation in infectiousness on disease emergence. Using contact tracing data from eight directly transmitted diseases, we show that the distribution of individual infectiousness around R0 is often highly skewed. Model predictions accounting for this variation differ sharply from average-based approaches, with disease extinction more likely and outbreaks rarer but more explosive. Using these models, we explore implications for outbreak control, showing that individual-specific control measures outperform population-wide measures. Moreover, the dramatic improvements achieved through targeted control policies emphasize the need to identify predictive correlates of higher infectiousness. Our findings indicate that superspreading is a normal feature of disease spread, and to frame ongoing discussion we propose a rigorous definition for superspreading events and a method to predict their frequency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Levin, S. A., Grenfell, B., Hastings, A. & Perelson, A. S. Mathematical and computational challenges in population biology and ecosystems science. Science 275, 334–343 (1997)
Becker, N. G. & Britton, T. Statistical studies of infectious disease incidence. J. R. Stat. Soc. B 61, 287–307 (1999)
Anderson, R. M. & May, R. M. Infectious Diseases of Humans: Dynamics and Control (Oxford Univ. Press, 1991)
Diekmann, O. & Heesterbeek, J. A. P. Mathematical Epidemiology of Infectious Diseases: Model Building, Analysis, and Interpretation (Wiley, Chichester, 2000)
Leo, Y. S. et al. Severe acute respiratory syndrome—Singapore, 2003. Morbid. Mortal. Wkly Rep. 52, 405–411 (2003)
Shen, Z. et al. Superspreading SARS events, Beijing, 2003. Emerg. Infect. Dis. 10, 256–260 (2004)
Dye, C. & Gay, N. Modeling the SARS epidemic. Science 300, 1884–1885 (2003)
Lipsitch, M. et al. Transmission dynamics and control of severe acute respiratory syndrome. Science 300, 1966–1970 (2003)
Riley, S. et al. Transmission dynamics of the etiological agent of SARS in Hong Kong: Impact of public health interventions. Science 300, 1961–1966 (2003)
Bauch, C. T., Lloyd-Smith, J. O., Coffee, M. & Galvani, A. P. Dynamically modeling SARS and other newly-emerging respiratory illnesses: past, present, future. Epidemiology 16, 791–801 (2005)
Koopman, J. Modeling infection transmission. Annu. Rev. Public Health 25, 303–326 (2004)
May, R. M. & Anderson, R. M. Transmission dynamics of HIV infection. Nature 326, 137–142 (1987)
Woolhouse, M. E. J. et al. Heterogeneities in the transmission of infectious agents: Implications for the design of control programs. Proc. Natl Acad. Sci. USA 94, 338–342 (1997)
Lloyd-Smith, J. O., Getz, W. M. & Westerhoff, H. V. Frequency-dependent incidence in models of sexually transmitted diseases: portrayal of pair-based transmission and effects of illness on contact behaviour. Proc. R. Soc. Lond. B 271, 625–634 (2004)
Anderson, R. M. et al. Epidemiology, transmission dynamics and control of SARS: the 2002–2003 epidemic. Phil. Trans. R. Soc. Lond. B 359, 1091–1105 (2004)
Gani, R. & Leach, S. Epidemiologic determinants for modeling pneumonic plague outbreaks. Emerg. Infect. Dis. 10, 608–614 (2004)
Becker, N. & Marschner, I. in Stochastic Processes in Epidemic Theory (eds Picard, P., Gabriel, J. P. & Lefevre, C.) 90–103 (Springer-Verlag, New York, 1990)
Becker, N. Analysis of Infectious Disease Data 48–59 (Chapman & Hall, London, 1989)
Bailey, N. T. J. The Mathematical Theory of Infectious Diseases 2nd edn, 263–265 (Griffin, London, 1975)
Keeling, M. J. & Grenfell, B. T. Disease extinction and community size: Modeling the persistence of measles. Science 275, 65–67 (1997)
Lloyd, A. L. Destabilization of epidemic models with the inclusion of realistic distributions of infectious periods. Proc. R. Soc. Lond. B 268, 985–993 (2001)
Meyers, L. A., Pourbohloul, B., Newman, M. E. J., Skowronski, D. M. & Brunham, R. C. Network theory and SARS: predicting outbreak diversity. J. Theor. Biol. 232, 71–81 (2005)
Boswell, M. T. & Patil, G. P. Random Counts in Models and Structures (ed. Patil, G. P.) 7–8 (Pennsylvania State Univ. Press, University Park, 1970)
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, New York, 2002)
Lloyd-Smith, J. O., Galvani, A. P. & Getz, W. M. Curtailing transmission of severe acute respiratory syndrome within a community and its hospital. Proc. R. Soc. Lond. B 270, 1979–1989 (2003)
Wallinga, J. & Teunis, P. Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am. J. Epidemiol. 160, 509–516 (2004)
Ha, L. D. et al. Lack of SARS transmission among public hospital workers, Vietnam. Emerg. Infect. Dis. 10, 265–268 (2004)
Park, B. J. et al. Lack of SARS transmission among healthcare workers, United States. Emerg. Infect. Dis. 10, 244–248 (2004)
Ferguson, N. M., Fraser, C., Donnelly, C. A., Ghani, A. C. & Anderson, R. M. Public health risk from the avian H5N1 influenza epidemic. Science 304, 968–969 (2004)
Farrington, C. P., Kanaan, M. N. & Gay, N. J. Branching process models for surveillance of infectious diseases controlled by mass vaccination. Biostatistics 4, 279–295 (2003)
Acknowledgements
We are grateful for comments and data suggestions from B. Bolker, J. Edmunds, N. Ferguson, A. Galvani, R. Gani, N. Gay, J. Gog, B. Grenfell, H. Hethcote, D. Heymann, A. Hubbard, N. Jewell, J. Lauer, R. May, T. Porco, C. Roth, D. Smith and B. Williams. We thank R. Gani for providing unpublished data from a previous publication, and L. Matthews for sharing work ahead of print. Our research was supported by the NSF, NIH-NIDA, the James S. McDonnell Foundation, the NSF/NIH Ecology of Infectious Disease Program, and the South African Centre for Epidemiological Modelling and Analysis (SACEMA). Author Contributions J.O.L.-S. and W.M.G. conceived the study. J.O.L.-S. collected and analysed outbreak data, conducted dynamic modelling, and drafted and revised the text. S.J.S. conducted formal analysis of branching processes and control measures. W.M.G. provided technical input on superspreading and control analyses. All authors contributed conceptually, and edited or commented on the text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains additional discussion of factors contributing to individual variation in infectiousness, methodological details on statistical and modelling analyses, and details of outbreak datasets and superspreading events. (PDF 435 kb)
Supplementary Figures
The file includes Supplementary Figures 1 to 4, with captions. The figures address the prediction of superspreading event frequency, further results on outbreak dynamics and control, and estimation of the dispersion parameter k with limited data. (PDF 322 kb)
Supplementary Table 1
A summary of results from our statistical analysis of uncontrolled outbreaks (corresponds to Figure 1a–c). (PDF 71 kb)
Supplementary Table 2
Detailed results from our statistical analysis of uncontrolled outbreaks (elaborating on Supplementary Table 1), and the analysis of data before and after control measures were applied in four outbreaks. (PDF 94 kb)
Rights and permissions
About this article
Cite this article
Lloyd-Smith, J., Schreiber, S., Kopp, P. et al. Superspreading and the effect of individual variation on disease emergence. Nature 438, 355–359 (2005). https://doi.org/10.1038/nature04153
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04153
This article is cited by
-
Transmission chains of the first local outbreak cause by Delta VariantB.1.617.2 COVID-19 in Guangzhou, Southern China
BMC Infectious Diseases (2024)
-
HPAIV outbreak triggers short-term colony connectivity in a seabird metapopulation
Scientific Reports (2024)
-
Frequency, kinetics and determinants of viable SARS-CoV-2 in bioaerosols from ambulatory COVID-19 patients infected with the Beta, Delta or Omicron variants
Nature Communications (2024)
-
Predicting nodal influence via local iterative metrics
Scientific Reports (2024)
-
Infectious Disease in the Workplace: Quantifying Uncertainty in Transmission
Bulletin of Mathematical Biology (2024)